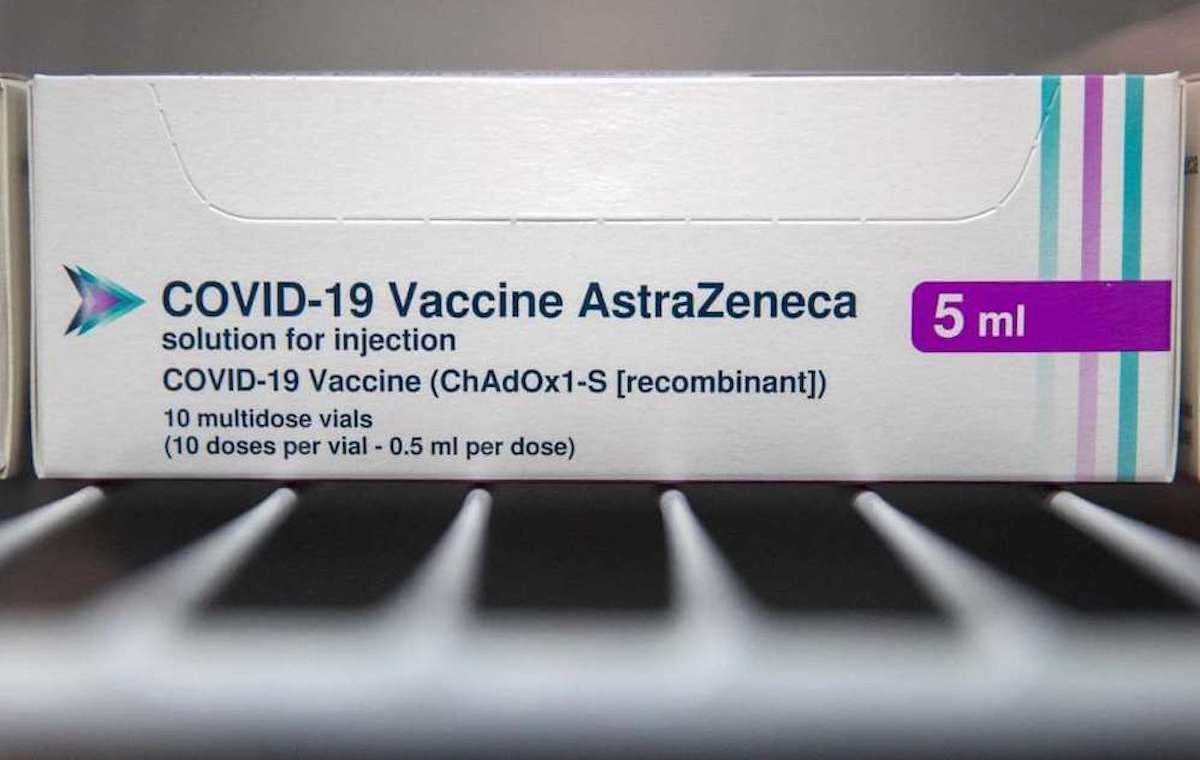
Il Oxford AstraZeneca vaccine is now getting closer and closer to official release. After the arrival of the first doses of the drug Modern in Italy last 11st January, now AstraZeneca made a formal request toEMA for the emergency marketing authorization of your vaccine (after it has already been approved in Britain). This was announced by the European drug agency itself with a tweet on its profile Twitter. If all goes smoothly, the AstraZeneca vaccine authorization could come next year 29st January. About this Ursula von der Leyen, Chairman of the European Commission, launched a hopeful tweet about it: "Once the vaccine receives a positive scientific opinion, we will work at full speed to authorize its use in Europe".
Vaccine Oxford AstraZeneca: differences with Pfizer and Moderna
In Europe, many have opted for the Oxford AstraZeneca vaccine, compared to those of Pfizer e Modern. The latter, being mRna vaccines, in fact, they need very low temperatures for storage (- 80 ° the first is -20 ° The second one). This also makes transportation and storage really difficult. The AstraZeneca vaccine, being a viral vector vaccine, can be transported more easily and stored at higher temperatures (2-8% for 30 days). As for the price, the Pfizer vaccine will cost (per dose) approximately 12 €, the Modern vaccine approx 15 € and AstraZeneca approx 2 €. Finally, very importantly, unlike Moderna e Pfizer / Biontech, for the Oxford AstraZeneca vaccine the recall will not be needed. In fact it will suffice a single dose per patient.
The arrival of the "European vaccine"In the Old Continent is now one step away from approval. Once you have received the green light from the EMA and theUe, in Italy they could reach up to 24 million doses by June. The Italian vaccine Reithera, developed in collaboration with the Spallanzani of Rome. Regarding the latter vaccine, the medical director of the Roman institute, Roberto Vaia, recently stated that with the production of 100 million doses of the ReiThera vaccine, Italy could become self-sufficient and solve any problem related to the demand for vaccines. Pending the homegrown vaccine, the approval of the AstraZeneca one is a very important step towards the end of the Covid-19 pandemic. Although the situation remains serious, the hope of a new life, in which the coronavirus is no longer scary, seems to be getting closer and closer.